Generating a synthetic ground truth for ophthalmic medical image analysis
- Degree programme: BSc in Informatik
- Authors: Emeline Lieberherr, Rayner Oswaldo Zorrilla Alfonzo
- Thesis advisor: Dr. Tiziano Ronchetti
- Expert: Dr. Harald Studer
- Year: 2021
As machine learning becomes ubiquitous in software engineering world, more and more projects are dedicated to implement this technology in the life sciences field. We propose a framework that processes ophthalmic OCT images (eye scan images) and predicts synthetic vitreal, retinal, choroidal and scleral semantic segmentations. In this context, we combine image augmentation techniques with a convolutional neural network presenting an encoder-decoder structure.
Introduction
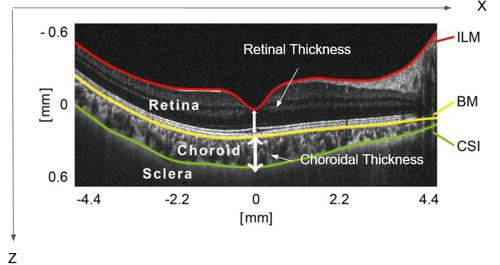
The choroid is the vascular layer of the eye. It contains connective tissues and is located between the sclera on the outside and the retina on the inside. Its thickness depends on several factors, including age, blood pressure and anatomic pathologies.
While in adults it has been found that the thickness of the choroid decreases with age, the same has not yet been demonstrated in children and adolescents. However, recent studies on choroidal development during childhood and adolescence contradict this finding. Several studies indicate that the choroidal thickness could be correlated with the prevalence of myopia. Therefore, the choroidal structure and thickness play a crucial role in monitoring the progression of myopia.
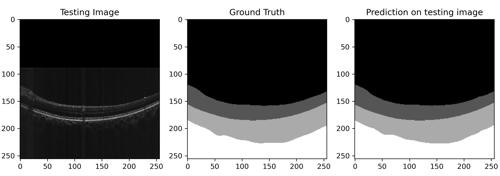
To measure changes in the choroidal thickness, it is necessary to detect all the different layers present in the ocular globe's periphery and their interfaces using OCT scans (eye scans). With the help of the segmentation of follow-up images it is possible to compare results over several years. However, a manual approach raises two important problems: firstly, the number of scans to be processed (layer identification) is considerable. Secondly, low contrast, loss of signal, and the presence of other image degradations makes it difficult to distinguish the sclera from that of the choroidal border. Therefore, a computerized solution, and more specifically a deep learning solution, is proposed to solve this problem.
Goal
The aim of this project is to develop a deep learning model capable of processing ophthalmic OCT images and predicting synthetic vitreal, retinal, choroidal and scleral semantic segmentation with a mean Jaccard coefficient of at least 95% (meaning that 95% of the pixel are correctly predicted). This will automate the measurements made on the scans and considerably shorten the time needed to produce a result, thus reducing the workload of the experts, and ensuring a high accuracy and standardization in the detection of the choroid.
Results
After training and cross validation, Model A had a mean Jaccard's coefficient of 96.78% on the validation set. The model had a mean of 97.80% sensitivity referring to the overall ability to accurately classify relevant pixels. On the other hand, the averaged specificity was 99.52%, which means that the model presents a high performance at rejecting pixels whenever they do not belong to a well-defined class. Model B presented a mean Jaccard's coefficient of 98.01%. Its sensibility was 96.72% while the averaged specificity 99.65%.
These results show that our framework is able to generate synthetic ground truth for ophthalmic medical image analysis.